- +49 201 43 70 97 0
- info@pakumed.de
- Mon - Fri: 8:30 - 17:00
TITAN PORT SPECIAL
TITAN-PORT F
This port system allows repeated access to the
- Cavum uteri (amnion)
- to the vascular system of the umbilical cord
- The use of the corresponding special cannulas is obligatory.
- hypoallergenic
- High biocompatibility
- particularly light (< 7 g)
- No chemical reactivity with injection solutions
- MR-conditionally safe up to 3 Tesla
- Secure twist and screw connection between chamber and catheter
- Suture holes for fixation to muscle fascia
- Compatible with non-coring port needles
- High pressure stability
- Self-closing special silicone
- Holds the port needle reliably in position
- Polyurethane: very flexible, but kink-resistant
- outstanding biocompatibility
- Suitable for long-term use
- free from latex, PVC and DEHP
- Length scaling for precise and safe implantation
- radiopaque
- with fixation ring (lock) to counteract dislocation in the uterus
- Catheter and port chamber are de-connectable
Description
Indication
Technical Data
SFN®- Port Needles
Instruction for Use
Youtube Channel
Customer Feedback
Description
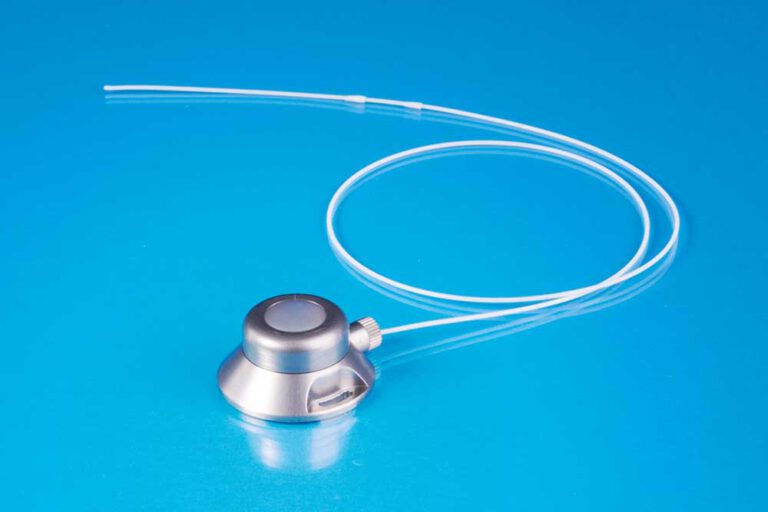
 The TITAN-PORT F is a fully implantable port catheter system. It consists of an injection chamber (port chamber) with a twist and screw closure mechanism and a self-closing silicone membrane and a polyurethane catheter. The system also comes with a special puncture cannula SFN 0720 S, a 20 G puncture cannula, an insertion cannula (18 G), instructions for use and an implantation card.
There are 2 indications for the Prenatal Port System TITAN-PORT F:
The TITAN-PORT F is a fully implantable port catheter system. It consists of an injection chamber (port chamber) with a twist and screw closure mechanism and a self-closing silicone membrane and a polyurethane catheter. The system also comes with a special puncture cannula SFN 0720 S, a 20 G puncture cannula, an insertion cannula (18 G), instructions for use and an implantation card.
There are 2 indications for the Prenatal Port System TITAN-PORT F:

Indication
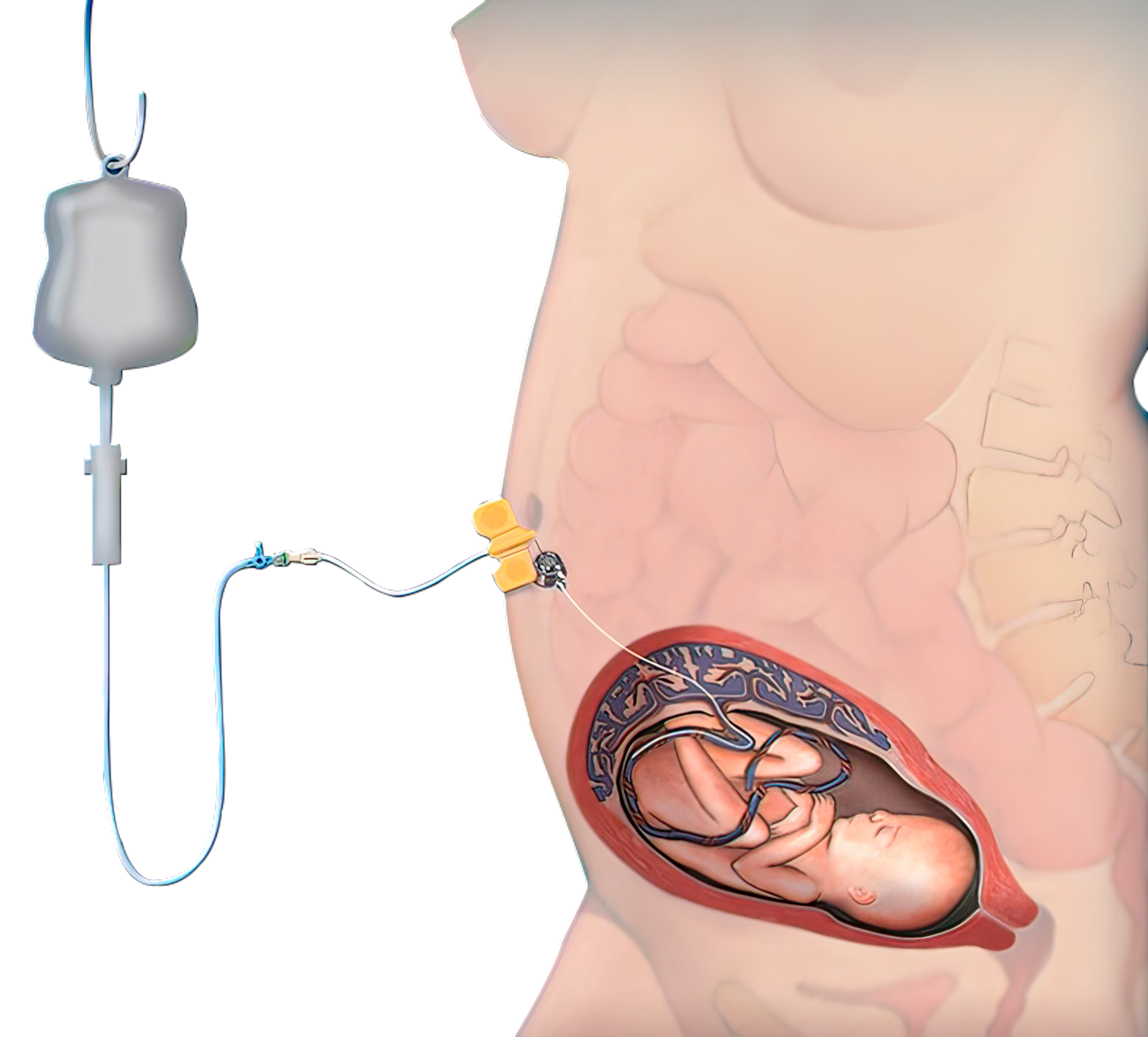
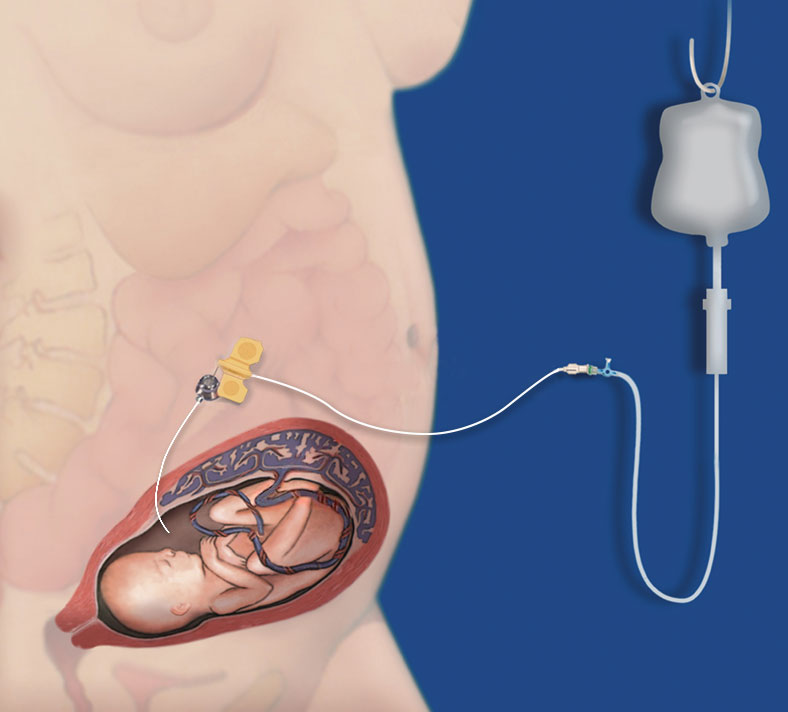
The TITAN-PORT F ensures the repeated access to:
A: Cavum uteri (Amnion)B: to the vascular system of the umbilical cord for e.g. the following applications:
A: Continuous amnioinfusion in preterm premature rupture of membranes (PPROM) with oligo-/anhydramnios
B: Infusion therapy and intrauterine nutrition in growth-restricted fetuses due to placental insufficiency (IUGR – intrauterine growth restriction)
The advantage is low risk of infection, simplified access and considerably improved quality of life for the patient as provided by a closed system.
Patient group:
A: PPROM: Patients between the 22nd and 30th week of pregnancy until delivery with the corresponding indication.
B: IUGR: Patients from the 24th to the 32nd week of pregnancy until birth with an appropriate indication with an estimated fetal weight of less than 1/10th of a percentile.
Operators: Implantation should only be carried out by experienced operators (with appropriate qualifications), usually neonatologists or gynaecologists at appropriate centres.
Intended purpose: The port catheter system is only used to fluids into or out of the patient’s vascular system. The product itself thus fulfils a physical property and has no medical, therapeutic effect.
Technical Data
| Art.No. | Port | Catheter | PZN | ||||||||
| Material | Basis | Height | Septum Ø | Weight | Material | AD | ID | Length | French | ||
| 111243 F (Fetal) | Titan | 21 mm | 9,8 mm | 8 mm | <10 g | Polyurethane | 0,64 mm | 0,42 mm | 400 mm | 1,92 | 08830327 |
SFN®- Port Needles
Only special non-coring port puncture needles (e.g., SFN® port needles or other suitable port needles) should be used to puncture the port membrane.
These needles exhibit a unique bevel at the tip. SFN 0720 S is recommended for standard use.
The use of special port needle prevents that holes or silicone particle are punched out, which could proceed into the bloodstream or plug the system. Each system contains a suitable needle.
| Art.No. | Ø | Length | Gauge | Extras | PZN |
| SFN 0720 S | 0,7 mm | 20 mm | 22 | bent with Luer/Lock and tubing | 07781615 |
Instruction for Use
Youtube Channel
Here you can find all application videos for our products.
Customer Feedback